Bioanalytical Services
Blood Biomarker Testing for Clinical Trials
Time-saving, cost-effective blood biomarker screening and monitoring tools for Alzheimer’s and other related dementia clinical studies
C2N Biopharma Clinical Research Services offers highly sensitive mass spectrometry-based identification, quantification, and monitoring of proteins, protein fragments (peptides), and other biomolecules implicated in human neurological diseases.
We assist our clients by extending their capabilities for using and developing novel fluid biomarkers and therapies for Alzheimer’s disease and other related brain disorders.
C2N’s assays have been used in over 150 Alzheimer’s disease and other research studies throughout the US and world. This includes landmark treatment and prevention trials involving disease-modifying therapies (DMTs) that are changing the trajectory of Alzheimer’s disease. C2N has ongoing collaborations with multi-national pharmaceutical and biotech companies, leading academic institutions, National Institute on Aging, Alzheimer’s Association, and other non-profits and consortiums. Over 50,000 Precivity-related biomarker measures have been reported through peer-reviewed publications, with many more manuscripts currently under review.
We also provide our Precivity™ line of tests as a service to accelerate the speed, lower the cost, and improve the efficiency of enrolling individuals into clinical trials; in addition, we apply our biomarkers to monitor for treatment effectiveness, study the epidemiology of disease, and understand new biological mechanisms of Alzheimer’s disease and related forms of dementia.
Potential Benefits of Partnering with C2N for Blood Testing in Clinical Trials
Time-saving, cost-effective blood biomarker screening and monitoring tools for Alzheimer’s and other related dementia clinical studies
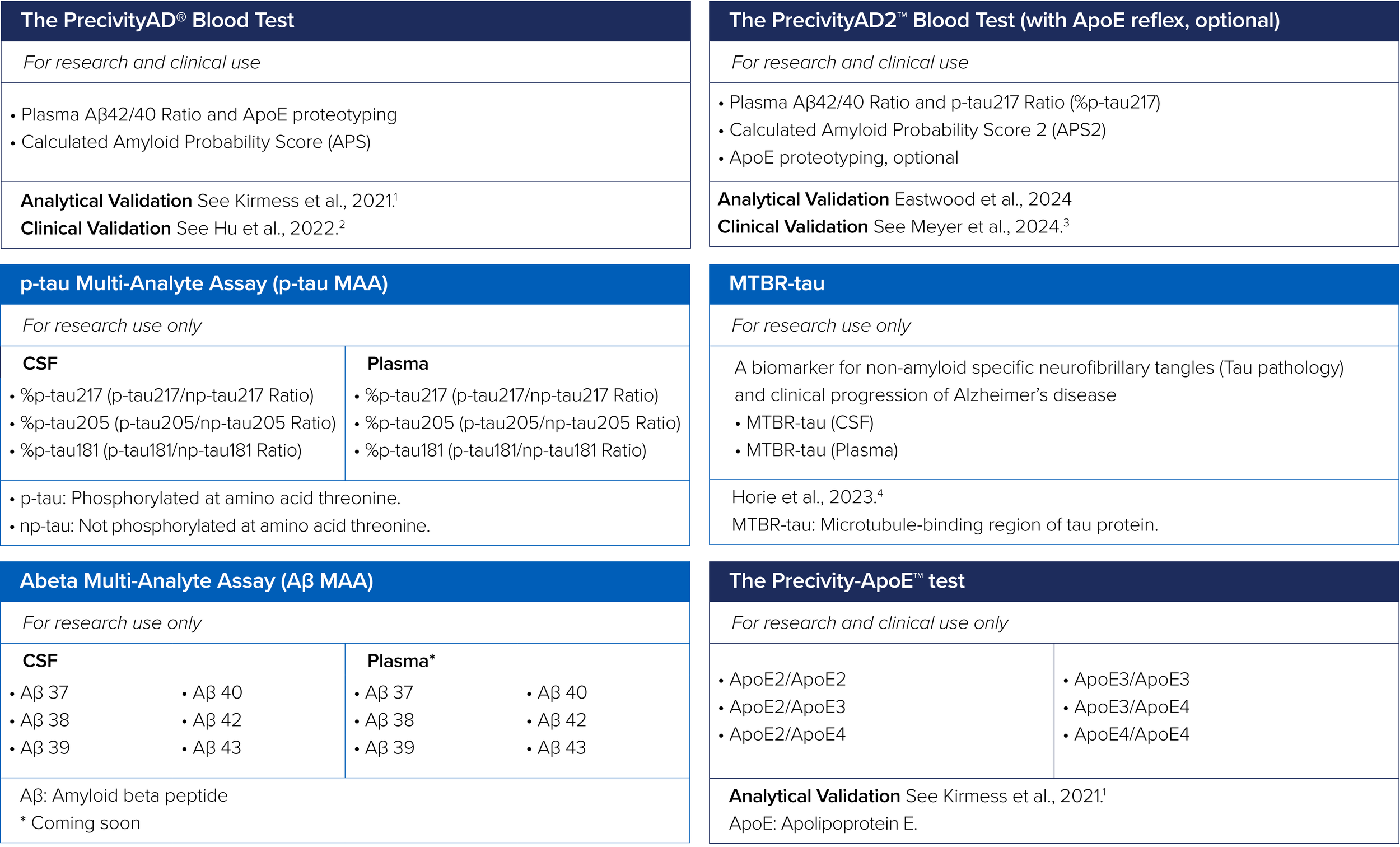
C2N’s Precivity™ portfolio of tests are being developed to address the entire spectrum of Alzheimer’s disease:
Risk Stratification | Early Detection | Disease Staging | Differential Diagnosis | Treatment Monitoring
Platform Methodology
Stable isotope labeled internal standard addition, immunoprecipitation followed by liquid chromatography-tandem mass spectrometry (LC-MS) specific identification and quantification of targeted protein and peptide biomarkers.
Quality Services
• Rapid turnaround time: biomarker values, Amyloid Probability Score (APS) values, eligibility information
• Customized data reporting to meet trial-specific and regulatory agency requirements
• Option to send eligibility reports to clinical sites
• Dedicated project management for study duration
Research Services Form
If you are interested in talking to C2N about a research project, please complete the form below. For all other inquiries regarding Bioanalytical services, please call 1.877.226.3424.